Digitalization does not mean a choice, but the inevitable course of enterprise development!
The construction of traceability system constitutes the infrastructure of industry digitization!
Getting through the supply chain, connecting the terminal and reaching the consumer are the goal of industry digitization!
Real and reliable traceability data is the bottleneck of industrial digitization!
Establishing a unique code standard from the production source is the key to build the whole-industry chain digital system!
Digitization value: establish all-contact connection, gather all-scenario data, and enhance all-element value!

Identification Cloud
Cloud coding platform, covering all coding service scenarios

Coding Cloud
Cloud code, GET product code to open a new way

Supply Chain Cloud
Supply chain lean management system,Full chain refined management expert

Terminal Cloud
Smart retail, millions of terminal online control

Marketing Cloud
How fast and good, brand code marketing easier

All-in-one Ceries Product
A variety of integrated equipment, enterprise automatic packaging preferred

Labeling Machine Series
High labeling precision, fast speed, with a variety of functions

Production Line Series
Perfect for pharmaceutical, fast moving consumer goods, industrial products multi-industry application scenarios
Why Choose Acctrue?
Chairman and President Xie Zhaohui


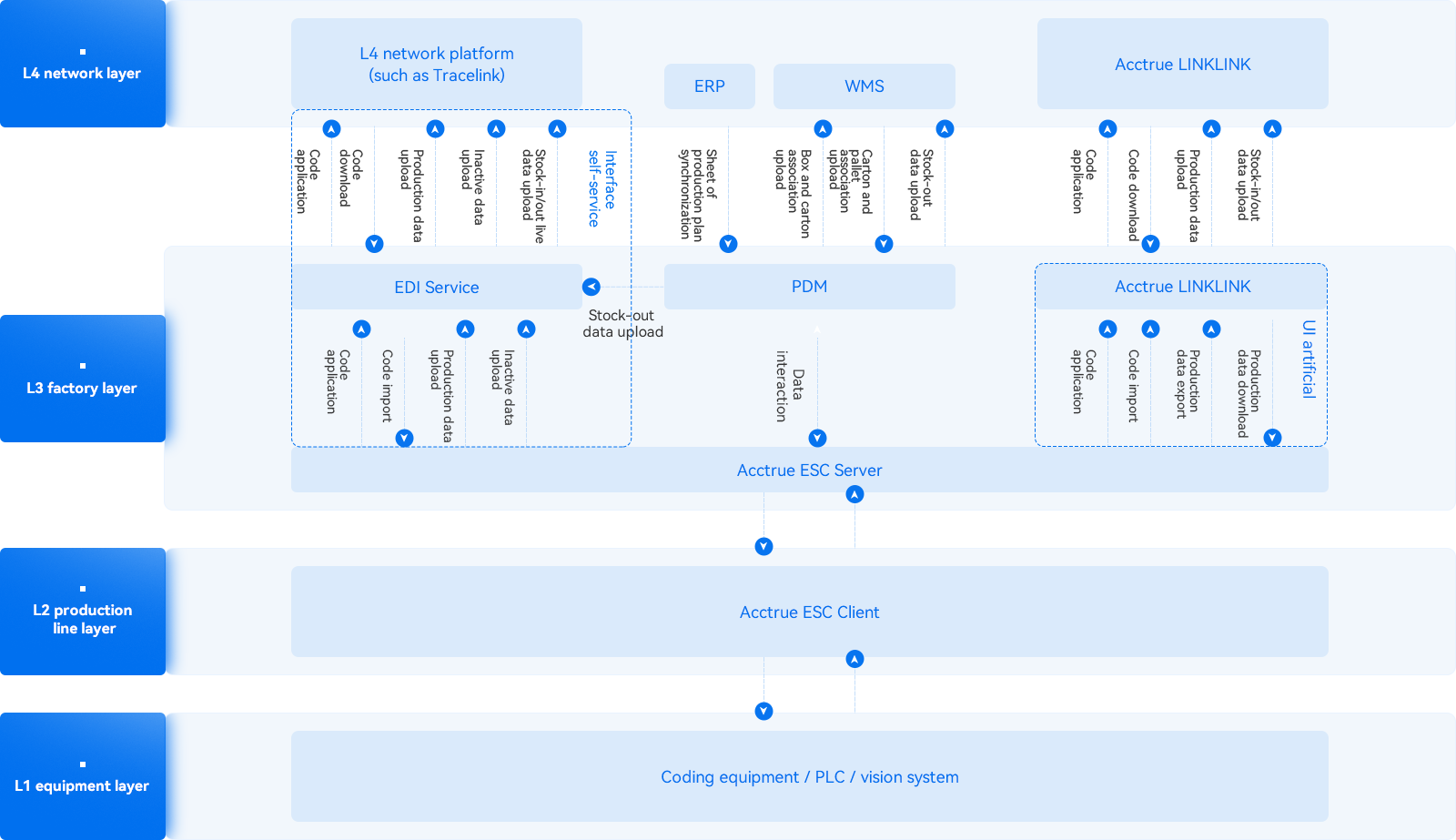
Production Line Coding
Acctrue Cloud Coding Solution

Anti-Counterfeiting and Anti-Channeling Goods
"Skyeye" Intelligent Anti-counterfeiting and Anti-channeling Solution

Supply Chain Track&Trace
Supply Chain Traceability Solution

Consumer Marketing
Solutions for HappyScan B2C Digital Marketing

Trace & Track Solution for Medicines Produced in China
Unanimous choice of 3000+ domestic and foreign pharmaceutical companies

Pharmacy Precision Marketing
It is the first in China to open WeChat "Super Entrance”, helping pharmaceutical enterprises win the terminal!

Track & Trace Compliance Solution Based on GS1 Standard for Drugs Exporting to China
Customers: Xi'an Janssen, Pfizer, Amgen, Bojian and Gilead

Drug Serialization Based on GS1 Standard
Successfully implemented 100+ imported medicine production lines, rich experience making us more trustworthy!

Medical Device UDI
Helped 100+ equipment enterprises to successfully implement UDI